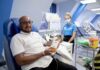
(Above) Saira Ali, 50, from Saltaire, was among the 720 volunteers who took part in vaccine trials at the University of Bradford after her husband became critically ill with Covid-19.
News that the Novovax Covid-19 vaccine – trialled in Bradford (England) – will shortly go before regulators, has been hailed as a “milestone for the UK” by the clinician leading the local trials.
Experts say it contains no animal products, a fact which could help persuade some minority groups who have ethical doubts over vaccines, to sign up for vaccination.
Professor Dinesh Saralaya, an honorary visiting professor at the University of Bradford, said news that the trials had been a success was “the highpoint of my career.”
The Novovax trials are the largest in the UK, with 15,200 people taking part, 726 of those from Bradford – tests were carried out at the University’s Digital Health Enterprise Zone (DHEZ).
Prof Saralaya said: “This is huge news for Bradford and the University. This is the largest Phase 3 covid-19 trial in the UK, with one in 20 of the 15,200 coming from our city.
“It is also the first vaccine to show efficacy against the Kent variant (89 per cent) and South African strain (50 per cent). It has an overall efficacy of 89.3 per cent. In the language of vaccines, if you can reach 70 per cent efficacy, that’s the primary goal, so this surpassed all expectations.”
No animal products in vaccine

Prof Saralaya also said the Novovax vaccine contained no animal products and, like the AstraZeneca/Oxford vaccine, was easy to transport.
“The vaccine is halal and kosher in that it contains no animal products. Novovax also has a very easy cold chain, like the Oxford vaccine, so it will be one of the vaccines which is taken door to door.”
He added: “For Bradford, this is stupendous. It means we have been part of the global race to find a cure for covid-19. I’m proud of my association with the University; I offer my thanks to Vice Chancellor Professor Shirley Congdon for allowing us to collaborate and use the DHEZ. I see this as a joint city effort between BTHFT, the University and the local federation of GPs.
“What will happen now is the vaccine will go before regulators. I hope it gets approved because what this means, with the UK buying 60m doses, is it will accelerate vaccinations and enable us to treat the entire population, so we protect the NHS and save lives. This is a milestone in the race to beat covid 19.”
Bradford architect Saira Ali was among those who took part in the trial, which included around 720 people from Bradford, some seven per cent of whom hailed from a BAME background. MP Naz Shah also volunteered.
‘Honour to contribute’
Dr Liz Breen, Director of the DHEZ, said: “We are honoured to have contributed to the Novavax trial within Bradford. The Digital Health Enterprise Zone prides itself on our collaborations with our local partners, to enhance healthcare and wellbeing delivery for our communities.
“The news of the Novavax efficacy is testament to the hard work of our patients, healthcare providers and supporting teams. We will continue to offer our support to Covid-19 research endeavours.”

Professor Congdon said: “The Novovax trials are a major part of the national fight against covid-19. The fact they were able to conduct those trials right here in Bradford, using the University’s facilities at the DHEZ, shows that we are at the forefront of global research. This is a proud day for the BTHFT, University, and the City Region.
The vaccine made by the US biotech firm will be produced in Stockton-on-Tees in the North East.
DHEZ is part of a £13m partnership led by the University of Bradford and the City of Bradford Metropolitan District Council, with £3.5m of funding from the Department of Business, Energy and Industrial Strategy. Leeds City Region Enterprise Partnership has supported DHEZ from the outset as the regional hub for digital health innovation.